There's a logical fallacy akin to a "what's good for one is good for all" mindset that is devastating when applied to surface treatment in adhesion processes. Polymers are rapidly being developed and synthesized for niche applications to push the limits of the current physical properties of materials. Polymers that are available today did not even exist a few years, or even months, ago. These different materials possess very particular molecular qualities that require distinct treatment approaches in order to compensate for their differences.
In order to utilize these cutting-edge plastic technologies, manufacturers need to be aware of the effect on the full material system – the baseline material, the adhesion, and the outcome of bond performance.
The chemical makeup of the baseline material surface is where it all begins, and controlling this aspect of the process can stop adhesion failure at the source. This is, however, often the most overlooked and least understood component of successful adhesion.
To illustrate how seemingly minor differences in material molecular structure can make or break a treatment process, let’s look at two of the most commonly used polymers in manufacturing; high-density polyethylene (HDPE) and Teflon (PTFE).
 |
 |
Revolutionize Your Manufacturing with Surface Quality Inspection Technology.
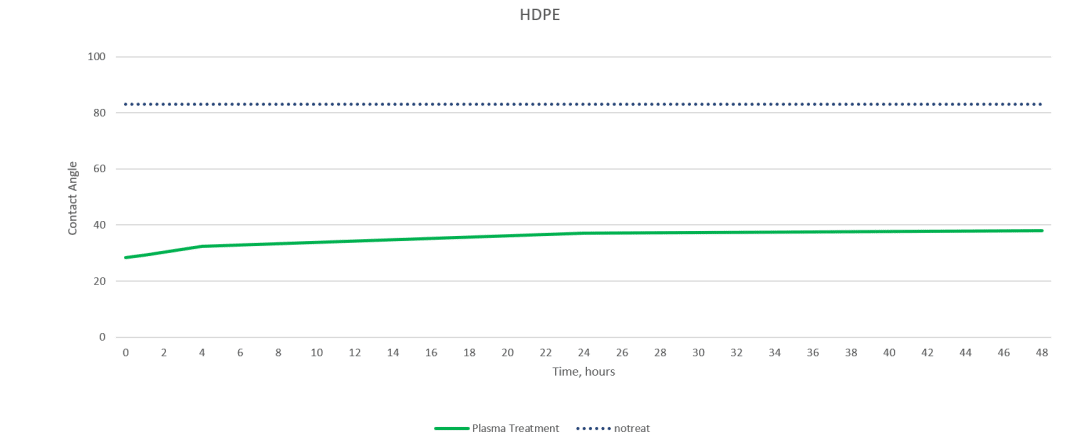
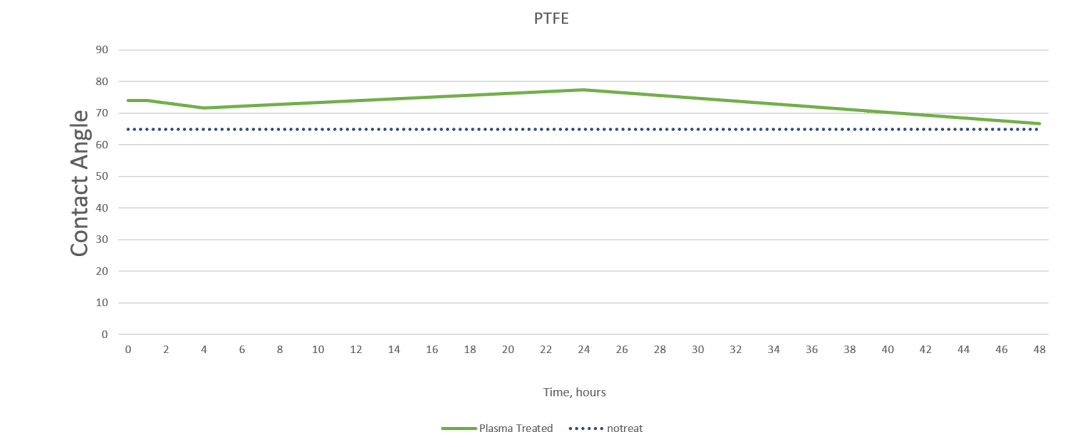
Plasmatreat, a manufacturer of plasma treatment systems, completed a study on various substrates to demonstrate how materials respond to the same preparation approach.
The charts below show contact angle measurements obtained using the Surface Analyst that gauges the effectiveness of Openair™ atmospheric plasma treatment on HDPE and PTFE. The measurement of the contact angle decreases as the surface energy (how amenable a material is to bonding) increases. Creating a surface with high surface energy can ensure a successful bond.
 |
 |
Images courtesy of Jonathan Schnur and Khoren Sahagian at Plasmatreat
As you can see, the treatment had little effect on the PTFE but brought the contact angle on the polyethylene down to the range where strong and stable adhesion is possible.
These two materials are just the tip of the iceberg when it comes to polymer differentiation. It’s crucial to match treatment to what the materials require. This treatment needs to be monitored, measured, and verified. This is not easy since the chemical structure of surfaces gets more complex.
The situation sounds dire, but it’s also possible to fully understand your materials, modify your preparation methods and ensure perfect adhesion.
Learn more about plasma treatment of polymers by viewing the webinar co-presented by Brighton Science's Founder and Chief Scientist, Giles Dillingham, and Plasmatreat's Khoren Sahagian.
View the full recorded webinar here.
Rethink your adhesion manufacturing processes with Surface Intelligence.




